As the Mobilise-D IMI-project reaches its conclusion, the coordinators have shared a powerful closing statement reflecting on the remarkable journey and achievements of the consortium, signed by Lynn Rochester, Professor of Human Movement Science, Institute of Neuroscience, Newcastle University, and Daniel Rooks, Executive Director, Clinical Excellence Head, Endpoint Management Program Discover& Profiling /Translational Medicine, Novartis.
In their closing remarks, the coordinators express their pride about the journey that has been and anticipation of the road ahead, acknowledging the significant developments made since the project’s inception and providing a look back at the project’s achievements, milestones, the dedication of its members, and the collaborative spirit that drove its success and innovation.
The closing statement also includes reflections on the challenges overcome and the lessons learned, underscoring the talent, hard work, and tenacity of the consortium members. They highlight the unique experience of being part of a multidisciplinary and multisectoral team, the transformative impact of the collective efforts, and simultaneously set the stage for future activities and advancements.
The full closing statement can be read below.
Stay connected with Mobilise-D for future updates and ongoing developments through our website, LinkedIn and YouTube channel.
Closing Statement
26th June 2024
Dear Mobilisers, Friday marks the final day of the work of the Mobilise-D consortium and it would be remiss not to share a final update as the co-ordinators to mark this milestone. We write this with mixed feelings – of pride in what we have accomplished together, with recognition that we have many more accomplishments ahead of us and in some ways – for the teams involved in WP4 and 6 – we are really just getting started!
From our perspective and many others, our work began in October 2017 and marks a near seven-year journey once we complete reporting for the EU. As said many times before – we have not only survived, in the face of so many challenges, we have excelled delivering not only on our core objectives but delivering many more insights. The talent, dedication, hard work, and tenacity of the consortium members has been the catalyst for this success.
Being part of a consortium such as Mobilise-D is a unique experience, both overwhelming and exciting in equal measure! The size and scale of the achievement was the result of each member’s contribution, our collective commitment, and the strength of our collaboration, something that was evident at the conference in Edinburgh. Mobilise-D has also for many of us provided a unique opportunity to work in a truly transformational project, that was multidisciplinary and multisectoral to its core. The chance to see our work reflected from this wide variety of perspectives is priceless and ultimately ensures that the work delivers the greatest impact. We hope you all enjoyed being part of this consortium as much as we did.
Here are some reflections on that journey – past, present, and future.
Looking back to where we started and why (2017): Mobility is both a marker of health and function and a core priority for people living with mobility limitations. However, prioritising mobility assessment and treatment was limited by a major barrier: no standardised, robust, reliable method to directly measure mobility continuously in the real-world and not knowing how digital mobility data connected to traditional mobility endpoints. The Mobilise-D consortium was formed to address this barrier and unmet need – to develop and deliver a technically sound solution to measure mobility in the real-world using a single wearable device, show this worked across multiple health conditions, demonstrate that the outcomes were clinically valid, and work towards adoption of these outcomes as the new standard for use in clinical trials and care. An objective was to highlight mobility globally as a core concept to be targeted. In April 2019, we commenced the Mobilise-D journey to tackle the many knowledge gaps and deliver on our aims and objectives.
So where are we at – and how did we do (2024)? The image below from the Mobilise-D Consortium Conference (March 2024) very succinctly summarises our many achievements. These are also set out in the Conference Brochure (Mobilise-D-Conference-Digital-Booklet.pdf). It is fair to say that collectively, we have removed barriers to adoption of mobility assessment through generic solutions for wide implementation that are standardised, acceptable, feasible, and scalable to enable cost effective, wide access for clinical research, care, and personalised healthcare. Our methods are endorsed and informed by the patients who will use them. Regulatory authorities endorse our methodological approaches giving confidence towards eventual adoption of digital mobility outcomes. We have educated, informed, and built the careers of the next generation of experts in digital mobility assessment. Our many achievements and lessons learnt are shared with the public through a public Deliverable (D7.6), which can be found on the Mobilise-D website (https://mobilise-d.eu/).
As identified above – there is still work to do in order to reflect on the results and publish the key findings from the clinical validation study. However, a solid foundation is in place to ensure that this will happen and the generous follow-on funding through the SUSTAIN Mobilise-D consortium, MJ Fox Foundation, and the formation of the Mobilise-D Network will ensure this is achieved.
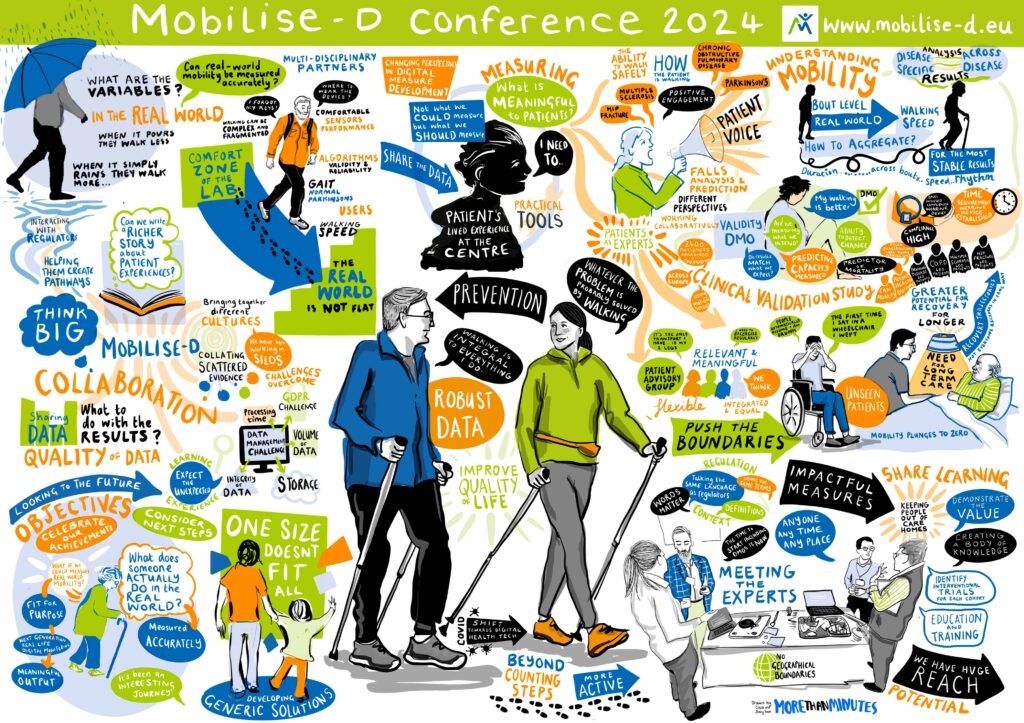
Looking to the future – what can we anticipate in the next 2-5 years? Collectively, our achievements will continue to deliver widespread impact across the development of new medicines and other therapeutics; technology improvement; patients and healthcare providers; clinical and technical research and sustainability; regulatory standards; and society. At an immediate level, releasing the algorithm for widespread use has allowed device manufacturers and companies to implement the Mobilise-D methods making them more widely available to all, with companies already integrating our algorithms and offering Mobilise-D DMOs in their services. This and the CVS data is anticipated to fill the knowledge gap and increase confidence leading to innovations in:
- Clinical research, through increased uptake in clinical trials by industry and academia. In turn, helping to generate the final evidence gap to support regulatory qualification – towards their use as secondary endpoints and potentially primary endpoints!
- Clinical care through increased interest from clinicians to consider how real-world mobility data could be used in clinical care pathways. Patients see this as an opportunity to help manage their own health conditions. Patients have already expressed interest to utilise Mobilise-D outcomes to enable more informed conversations with their health care professionals and provide greater agency over their care – prioritizing areas that mater to them and their quality of life. This will form the building blocks to generate the evidence for HTA bodies for implementation in clinical care pathways and the needed funding for this.
In Mobilise-D we created the conditions for our methods, knowledge, and people (Mobilisers 😊) to thrive and grow. To take the theme of the conference, this has provided fertile ground that will ensure a lasting legacy of our work. SUSTAIN Mobilise-D is a key example of the confidence and value of the consortium through an ongoing commitment by EFPIA partners to continue to leverage knowledge and insights from the data and facilitate the implementation of best practices over all aspects of the DMO process. The Mobilise-D Network will also continue this legacy providing a knowledge sharing and discussion forum to advance digital mobility assessment widely.
Future Mobilise-D communication and final comments: We will be releasing information on how to continue to access data and results so look out for continued communication from the consortium. The Mobilise-D results will continue to be shared and promoted through our LinkedIn channels, YouTube, and the Mobilise-D website, and in due course we will prepare a press release that we will share.
We would like to extend thanks to: the Mobilise-D consortium members for your continued dedication and contribution; to the Project Executive for continued leadership – helping to steer us towards the outcomes we have achieved; to the Patient and Public Advisory group – without whom our work would be so much less, your insights and collaborative approach have delivered fit for purpose tools and knowledge, and shone a light on what is possible to achieve together if the right environment is created; to our Scientific Advisory Board for their wise counsel over the years; and to the International Study Steering Committee – who provided ongoing oversight of the clinical validation study ensuring the work continued to the high stand required.
Finally – the reins are now passed into the capable hands of Dr Dan Rooks and Professor Brian Caulfield to lead the SUSTAIN Mobilise-D consortium! Together with the Mobilise-D Network (to be constituted in the autumn – more to follow on this) we can look forward to many more advances in knowledge. The sky’s the limit! Please continue to follow the progress on the Mobilise-D website and social communication channels. It has been a privilege serving as the co-ordinators for this outstanding consortium. Wishing you all the best of luck in future endeavours personal and professional. We look forward to seeing many of you in the future, to see how the work is adopted, and what new insights this delivers for patients.
Lynn Rochester and Dan Rooks
Mobilise-D Project Co-ordinators