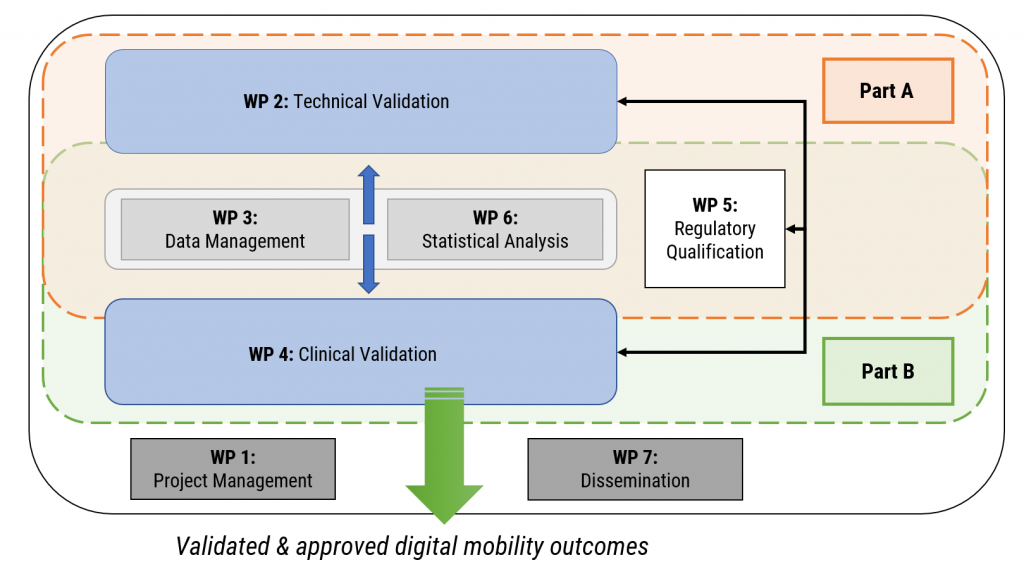
WP1 dealt with overall Project Coordination, Project Management, Financial Management, and Risk Management.
In addition, it focused on the development of strategies and long-term project plans, and ensured that work complied with national and EU Health and Safety regulations and Ethical Guidelines.